post revised 18 June 2019
The IUPAC Periodic Table of the Elements and Isotopes (IPTEI) for the Education Community co-authored by N. E. Holden, T. B. Coplen, J. K. Böhlke, L. V. Tarbox, J. Benefield, J. R. de Laeter, P. G. Mahaffy, G. O’Connor, E. Roth, D. H. Tepper, T. Walczyk, M. E. Wieser, S. Yoneda and published in Pure and Applied Chemistry, 90(12), 1833–2092 (2018) is an authoritative resource for the educational community. See https://doi.org/10.1515/pac-2015-0703.
Abstract: The IUPAC Periodic Table of the Elements and Isotopes (IPTEI) was created to familiarize students, teachers, and non-professionals with the existence and importance of isotopes of the chemical elements. The IPTEI is modeled on the familiar Periodic Table of the Chemical Elements. The IPTEI is intended to hang on the walls of chemistry laboratories and classrooms.
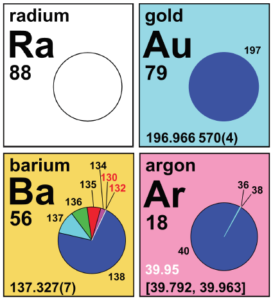 Each cell of the IPTEI provides the chemical name, symbol, atomic number, and standard atomic weight of an element. Color-coded pie charts in each element cell display the stable isotopes and the relatively long-lived radioactive isotopes having characteristic terrestrial isotopic compositions that determine the standard atomic weight of each element. The background color scheme of cells categorizes the 118 elements into four groups: (1) white indicates the element has no standard atomic weight, (2) blue indicates the element has only one isotope that is used to determine its standard atomic weight, which is given as a single value with an uncertainty, (3) yellow indicates the element has two or more isotopes that are used to determine its standard atomic weight, which is given as a single value with an uncertainty, and (4) pink indicates the element has a well-documented variation in its atomic weight, and the standard atomic weight is expressed as an interval.
Each cell of the IPTEI provides the chemical name, symbol, atomic number, and standard atomic weight of an element. Color-coded pie charts in each element cell display the stable isotopes and the relatively long-lived radioactive isotopes having characteristic terrestrial isotopic compositions that determine the standard atomic weight of each element. The background color scheme of cells categorizes the 118 elements into four groups: (1) white indicates the element has no standard atomic weight, (2) blue indicates the element has only one isotope that is used to determine its standard atomic weight, which is given as a single value with an uncertainty, (3) yellow indicates the element has two or more isotopes that are used to determine its standard atomic weight, which is given as a single value with an uncertainty, and (4) pink indicates the element has a well-documented variation in its atomic weight, and the standard atomic weight is expressed as an interval.
An element-by-element review accompanies the IPTEI and includes a chart of all known stable and radioactive isotopes for each element. Practical applications of isotopic measurements and technologies are included for the following fields: forensic science, geochronology, Earth-system sciences, environmental science, and human health sciences, including medical diagnosis and treatment.
{more}
– Follow original IUPAC project 2007-038-3-200 and follow-up project 2014-024-1-200
– Visit iupac.org Periodic Table of the elements https://iupac.org/what-we-do/periodic-table-of-elements/
– Read Why Isotopes Matter! https://iupac.org/100/stories/why-isotopes-matter/
– Explore the Interactive version at https://www.isotopesmatter.com/
{back to Periodic Table}
