Earlier versions of the Periodic Table released since 1999
- 4 May 2022 [current version]
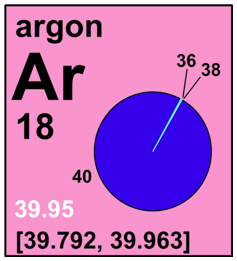
- 1 Dec 2018 [pdf file, letter size or A4 or A3 (PDF) version] including the most recent updates released in June 2018 by the IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW) (see related News, released 5 June 2018), and specifically for argon, the assignment of an interval for the new standard atomic weight which reflects the common occurrence of variations in the atomic weights of the element in normal terrestrial materials. An interval in square brackets provides the lower and upper bounds of the standard atomic weight for that element. For users needing an atomic-weight value for an unspecified sample with disregard to the uncertainty, the conventional values are provided. No values are listed for elements which lack isotopes with a characteristic isotopic abundance in natural terrestrial samples. See PAC for more details or visit Commission II.1. Reprint as back cover tear off Chemistry International Jan-Mar 2019 issue.
- 28 Nov 2016 [pdf file, letter size or A4 or A3 (PDF) version] including the added elements 113, 115, 117, and 118 with their names and symbols (see related News), the standard atomic weights 2013 (abridged to five significant digits) and the conventional atomic weights as published in PAC Vol. 88, No.3, pp. 265-291 (https://dx.doi.org/10.1515/pac-2015-0305), and for ytterbium, the standard atomic weight updated following the 2015 review (see related News). An interval in square brackets provides the lower and upper bounds of the standard atomic weight for that element. For users needing an atomic-weight value for an unspecified sample with disregard to the uncertainty, the conventional values are provided. No values are listed for elements which lack isotopes with a characteristic isotopic abundance in natural terrestrial samples; reprint as back cover tear off Chemistry International Jan-Mar 2017 issue.
- 8 Jan 2016 [pdf file – 109 KB] including Uut, Uup, Uus, and Uuo and following the announcement on 30 Dec 2015 of the discoveries of four new chemical elements. This table includes the recently added elements 113, 115, 117, and 118 with their temporary names and symbols (see related News, released 30 Dec 2015), the standard atomic weights 2013 (abridged to four significant digits and as deduced from PAC 88, No.3 (2016) https://dx.doi.org/10.1515/pac-2015-0305), and for ytterbium, the standard atomic weight updated following the 2015 review (see related News, released 24 Aug 2015).
- 1 May 2013 [pdf file – 88 KB] including the standard atomic weights 2011 abridged to four significant digits (as published in PAC 85, table 4, p. 1068; doi.org/10.1351/PAC-REP-13-03-02; reprint as back cover tear off Chemistry International Jul-Aug 2013 issue.
- 11 June 2012 [pdf file – 88KB] including Fl and Lv (The IUPAC recommendations of the names and symbols of the elements with atomic numbers 114 and 116 is to appear in PAC July 2012; https://dx.doi.org/10.1351/PAC-REC-11-12-03); CI tear off Jul-Aug 2012 issue
- Jul-Aug 2011 — Periodic Table of the Isotopes — [pdf file – 928KB] – see related Chem Int article ; see Interactive version release 16 Aug 2016
- 21 January 2011 [pdf file – 135KB] including the standard atomic weights 2009 abridged to four significant digits (as published in PAC 83, table 4, p. 387 [doi.org/10.1351/PAC-REP-10-09-14]) – see related Chem Int feature (Mar-Apr 2011 issue)
- 19 February 2010 [pdf file – 196KB] including the standard atomic weights 2007 abridged to four significant digits (as published in PAC 81, table 4, p. 2141 [doi:10.1351/PAC-REP-09-08-03]) and the latest named element copernicium > see CI notice
- 23 August 2007 [pdf file – 201KB] (version date 23 August 2007) including the 2007 revised standard atomic weights of 5 chemical elements > see release published in Chem. Int. Nov 2007
- 22 June 2007 [pdf file – 28KB] sized to print on A4 and US letter paper, including the 2005 revised standard atomic weights of 16 chemical elements >see release published in Chem. Int. Nov-Dec 2005
- 3 Oct 2005 [pdf file – 17KB] or see CI tear off version May-June 2006 issue [pdf file – 68KB]
- 4 Feb 2005 [pdf file – 17KB]; CI tear off Mar-Apr 2005 issue [pdf file – 87KB]
- 1 Nov 2004 [pdf file – 17KB]
- 7 Nov 2003 [pdf file – 17KB]; CI Jan-Feb 2004 issue tear-off table [pdf file – 474KB], and a brief historical review of the table, see Chem. Int. 2004, Jan , p. 8
In this version below, each element is keyed to a color matching the time of its discovery
|
| 1 | 18 | ||||||||||||||||
| 1 H 1.0079 |
2 | 13 | 14 | 15 | 16 | 17 | 2 He 4.0026 |
||||||||||
| 3 Li 6.941 |
4 Be 9.0122 |
5 B 10.811 |
6 C 12.011 |
7 N 14.007 |
8 O 15.999 |
9 F 18.998 |
10 Ne 20.180 |
||||||||||
| 11 Na 22.990 |
12 Mg 24.305 |
3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 Al 26.982 |
14 Si 28.086 |
15 P 30.974 |
16 S 32.065 |
17 Cl 35.453 |
18 Ar 39.948 |
| 19 K 39.098 |
20 Ca 40.078 |
21 Sc 44.956 |
22 Ti 47.867 |
23 V 50.942 |
24 Cr 51.996 |
25 Mn 54.938 |
26 Fe 55.845 |
27 Co 58.933 |
28 Ni 58.693 |
29 Cu 63.546 |
30 Zn 65.38 |
31 Ga 69.723 |
32 Ge 72.64 |
33 As 74.922 |
34 Se 78.96 |
35 Br 79.904 |
36 Kr 83.798 |
| 37 Rb 85.468 |
38 Sr 87.62 |
39 Y 88.906 |
40 Zr 91.224 |
41 Nb 92.906 |
42 Mo 95.96 |
43 Tc – |
44 Ru 101.07 |
45 Rh 102.91 |
46 Pd 106.42 |
47 Ag 107.87 |
48 Cd 112.41 |
49 In 114.82 |
50 Sn 118.71 |
51 Sb 121.76 |
52 Te 127.60 |
53 I 126.90 |
54 Xe 131.29 |
| 55 Cs 132.91 |
56 Ba 137.33 |
57-71 | 72 Hf 178.49 |
73 Ta 180.95 |
74 W 183.84 |
75 Re 186.21 |
76 Os 190.23 |
77 Ir 192.22 |
78 Pt 195.08 |
79 Au 196.97 |
80 Hg 200.59 |
81 Tl 204.38 |
82 Pb 207.2 |
83 Bi 208.98 |
84 Po – |
85 At – |
86 Rn – |
| 87 Fr – |
88 Ra – |
89-103 | 104 Rf – |
105 Db – |
106 Sg – |
107 Bh – |
108 Hs – |
109 Mt – |
110 Ds – |
111 Rg – |
112 Cn – |
113 Nh – |
114 Fl – |
115 Mc – |
116 Lv – |
117 Ts – |
118 Og – |
| La 150.36 | La 150.36 | La 150.36 |
57 La 138.91 |
58 Ce 140.12 |
59 Pr 140.91 |
60 Nd 144.24 |
61 Pm – |
62 Sm 150.36 |
63 Eu 151.96 |
64 Gd 157.25 |
65 Tb 158.93 |
66 Dy 162.50 |
67 Ho 164.93 |
68 Er 167.26 |
69 Tm 168.93 |
70 Yb 173.05 |
71 Lu 174.97 |
| La 150.36 | La 150.36 | La 150.36 |
89 Ac – |
90 Th 232.04 |
91 Pa 231.04 |
92 U 238.03 |
93 Np – |
94 Pu – |
95 Am – |
96 Cm – |
97 Bk – |
98 Cf – |
99 Es – |
100 Fm – |
101 Md – |
102 No – |
103 Lr – |
Notes
– Standard atomic weights last revised based on the 2005 review published in Pure Appl. Chem. 78(11), 2051-2066, 2006 (doi.org/10.1351/pac200678112051) and Aug 2007 release (also in Chem. Int. Nov 2007), and here reported to 5 significant figures. Elements with no reported values in the table above have no stable nuclides (alternative tables might display mean relative atomic masses or mass numbers for an arbitrarily selected unstable nuclide of that chemical element). However, three such elements -Th, Pa, and U- have a characteristic terresterial isotopic composition, and for these an atomic weight is tabulated.
A similar table, commemorative of IUPAC 80 Years of Service to Chemistry was produced as a laminated postcard and distributed with the Nov. 2000 issue of Chemistry International
|
Symbol |
name |
Symbol | name | Symbol | name | |||
| Ac | actinium | Gd | gadolinium | Pd | palladium | |||
| Ag | silver* | Ge | germanium | Pm | promethium | |||
| Al | aluminium+ | H | hydrogen | Po | polonium | |||
| Am | americium | He | helium | Pr | praseodymium | |||
| Ar | argon | Hf | hafnium | Pt | platinum | |||
| As | arsenic | Hg | mercury* | Pu | plutonium | |||
| At | astatine | Ho | holmium | Ra | radium | |||
| Au | gold* | Hs | hassium | Rb | rubidium | |||
| B | boron | I | iodine | Re | rhenium | |||
| Ba | barium | In | indium | Rf | rutherfordium | |||
| Be | beryllium | Ir | iridium | Rg | roentgenium | |||
| Bh | bohrium | K | potassium* | Rh | rhodium | |||
| Bi | bismuth | Kr | krypton | Rn | radon | |||
| Bk | berkelium | La | lanthanum | Ru | ruthenium | |||
| Br | bromine | Li | lithium | S | sulfur | |||
| C | carbon | Lr | lawrencium | Sb | antimony* | |||
| Ca | calcium | Lu | lutetium | Sc | scandium | |||
| Cd | cadmium | Lv | livermorium | Se | selenium | |||
| Ce | cerium | Mc | moscovium | Sg | seaborgium | |||
| Cf | californium | Md | mendelevium | Si | silicon | |||
| Cl | chlorine | Mg | magnesium | Sm | samarium | |||
| Cm | curium | Mn | manganese | Sn | tin* | |||
| Cn | copernicium | Mo | molybdenum | Sr | strontium | |||
| Co | cobalt | Mt | meitnerium | Ta | tantalum | |||
| Cr | chromium | N | nitrogen | Tb | terbium | |||
| Cs | caesium+ | Na | sodium* | Tc | technetium | |||
| Cu | copper* | Nb | niobium | Te | tellurium | |||
| Db | dubnium | Nd | neodymium | Th | thorium | |||
| Ds | darmstadtium | Ne | neon | Ti | titanium | |||
| Dy | dysprosium | Nh | nihonium | Tl | thallium | |||
| Er | erbium | Ni | nickel | Tm | thulium | |||
| Es | einsteinium | No | nobelium | Ts | tennessine | |||
| Eu | europium | Np | neptunium | U | uranium | |||
| F | fluorine | O | oxygen | V | vanadium | |||
| Fe | iron* | Og | oganesson | W | tungsten* | |||
| Fl | flerovium | Os | osmium | Xe | xenon | |||
| Fm | fermium | P | phosphorus | Y | yttrium | |||
| Fr | francium | Pa | protactinium | Yb | ytterbium | |||
| Ga | gallium | Pb | lead* | Zn | zinc | |||
| Zr | zirconium |
* Some element symbols derive from ancient names; Ag derives from argentum, Au from aurum, Cu from cuprum, Fe from ferrum, Hg from hydrargyrum, K from kalium, Na from natrium, Pb from plumbum, Sb from stibium, Sn from stannum, and W from wolfram.
+ Alternative spellings commonly used are: ‘aluminum’ for Al and ‘cesium’ for Cs.^ page top
Page last modified 14 Feb 2018.
Copyright ©1997-2018 International Union of Pure and Applied Chemistry.