5 June 2018 – The IUPAC Commission on Isotopic Abundances and Atomic Weights (CIAAW) met under the chairmanship of Dr. Juris Meija, at the University of Groningen, the Netherlands, in September 2017. Following its meeting, the Commission recommended changes to the standard atomic weights of 14 chemical elements.
1.) The standard atomic weights of argon and iridium have been changed based on recent determinations and evaluations of terrestrial isotopic abundances. For argon, assignment of an interval for the new standard atomic weight reflects the common occurrence of variations in the atomic weights of the element in normal terrestrial materials. The standard atomic weights of the other 12 elements, all having a single stable isotope, have been revised based on the new assessment of their atomic masses endorsed by the International Union of Pure and Applied Physics.
 aluminium (aluminum): from 26.981 5385(7) to 26.981 5384(3)
aluminium (aluminum): from 26.981 5385(7) to 26.981 5384(3)
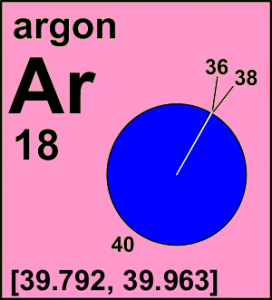
argon: from 39.948(1) to [39.792, 39.963]
cobalt: from 58.933 194(4) to 58.933 194(3)
gold: from 196.966 569(5) to 196.966 570(4)
holmium: from 164.930 33(2) to 164.930 328(7)
iridium: from 192.217(3) to 192.217(2)
manganese: from 54.938 044(3) to 54.938 043(2)
niobium: from 92.906 37(2) to 92.906 37(1)
praseodymium: from 140.907 66(2) to 140.907 66(1)
protactinium: from 231.035 88(2) to 231.035 88(1)
rhodium: from 102.905 50(2) to 102.905 49(2)
terbium: from 158.925 35(2) to 158.925 354(8)
thulium: from 168.934 22(2) to 168.934 218(6)
yttrium: from 88.905 84(2) to 88.905 84(1)
The reported uncertainties of the standard atomic weights are such that the atomic-weight values of normal materials are expected to lie in the given interval with great certitude. There are three broad groups of elements depending on what is the main cause of the uncertainty of their standard atomic weights: (1) well-documented natural variations of isotopic abundances, (2) our ability to determine the isotopic abundances, and (3) our ability to precisely determine the atomic masses of the isotopes. Elements in the first category are distinguished by an interval standard atomic weight. For instance, the standard atomic weight of argon, [39.792, 39.963], indicates that atomic-weight values of argon in normal materials are expected to be from 39.792 to 39.963. For iridium, which falls into second category, the standard atomic weight of iridium, 192.217(2), indicates that atomic-weight values of iridium in normal materials are expected to be from 192.215 to 192.219. For all other elements above the uncertainty of the standard atomic weight is the result of the measurement precision of their atomic mass. As an example, the standard atomic weight of holmium, 164.930 328(7), indicates that the best measurements result in a value from 164.930 321 to 164.930 335.
2.) The CIAAW also notes that the international isotopic reference material LSVEC lithium carbonate which (together with NBS 19) is used to realize the carbon isotope delta scale (VPDB), is able to absorb carbon dioxide from air. This process changes the carbon isotope ratio in LSVEC with time. Given that LSVEC is unsuitable as a reference material for carbon isotope ratio analysis, its use is no longer recommended. Carbon isotope delta measurements must still be normalized to the VPDB scale using at least two suitable international reference materials selected by the users as appropriate.
These changes and considerations will be published in Pure and Applied Chemistry and further details can be found online at the website of the IUPAC Commission on Isotopic Abundances and Atomic Weights (www.ciaaw.org).
On behalf of the CIAAW
Juris Meija, Chairman CIAAW
Thomas Prohaska, Secretary CIAAW; Johanna Irrgeher, acting Secretary CIAAW (Sep 2017)
(release also published in CI Oct 2018, p. 23; https://doi.org/10.1515/ci-2018-0409)
About this … see
Chemistry World, 7 June 2018 News ‘Noble gas’s days of having a fixed atomic weight argon‘
