24 AUGUST 2015 – The IUPAC Commission on Isotopic Abundances and Atomic Weights (II.1) met under the chairmanship of Dr. Juris Meija, at the University of Natural Resources and Life Sciences Vienna, Austria, prior to the 48th IUPAC General Assembly in Busan, Korea, in August 2015. Following its meeting, the Commission recommended a change to the standard atomic weight of ytterbium. The IUPAC Bureau, at its meeting on 14 August, approved this change.
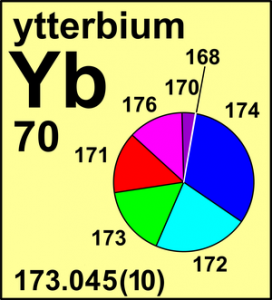
The standard atomic weight of ytterbium has been changed based on a recent measurement of terrestrial isotopic abundances from 173.054(5) to 173.045(10). For ytterbium, which was first obtained in a pure state just some 50 years ago, only two calibrated measurements of its isotopic composition have been ever made. Both of these measurements differ from one another. Ytterbium exemplifies the situation that is also true for many other elements: well-documented isotope ratio measurements are still needed.
In addition to revising standard atomic weights, CIAAW discussed the uncertainty interpretation of standard atomic weights and resolved to endorse probabilistic interpretation of standard atomic weights using various probability density functions, including the uniform probability distribution as outlined in the Eurachem/CITAC Guide when no specific knowledge is available about the isotopic composition of a material of interest. In all cases, probability distributions describe the lack of knowledge about isotopic compositions of particular materials or classes of materials, and not the actual distribution of natural abundances.
These changes and considerations will be published in the upcoming release of the “Atomic Weights of the Elements 2015”, which will appear in Pure and Applied Chemistry. The revised values for standard atomic weights can also be found on-line at the website of the IUPAC Commission on Isotopic Abundances and Atomic Weights (www.ciaaw.org).
The importance of determining precise atomic weights has long been recognized, resulting in the creation of the International Atomic Weights Committee in 1899. IUPAC has overseen the periodic evaluation and dissemination of standard atomic-weight values since its formation in 1919.
Contact:
Dr. Juris Meija <[email protected]>, Chair of IUPAC Commission II.1
