Metrology, the science of measurement, is part of the essential but largely hidden infrastructure of the modern world. We need it for high-technology manufacturing, human health and safety, the protection of the environment, global climate studies, information transfer and the basic science that underpins all these. Highly accurate measurements are no longer the preserve of only the physical sciences and engineering. The International System of Units, the SI (Système International d’unités), provides the internationally agreed means by which we make such measurements.
Metrology, the science of measurement, is part of the essential but largely hidden infrastructure of the modern world. We need it for high-technology manufacturing, human health and safety, the protection of the environment, global climate studies, information transfer and the basic science that underpins all these. Highly accurate measurements are no longer the preserve of only the physical sciences and engineering. The International System of Units, the SI (Système International d’unités), provides the internationally agreed means by which we make such measurements.
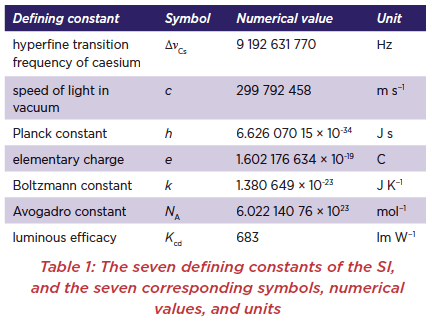
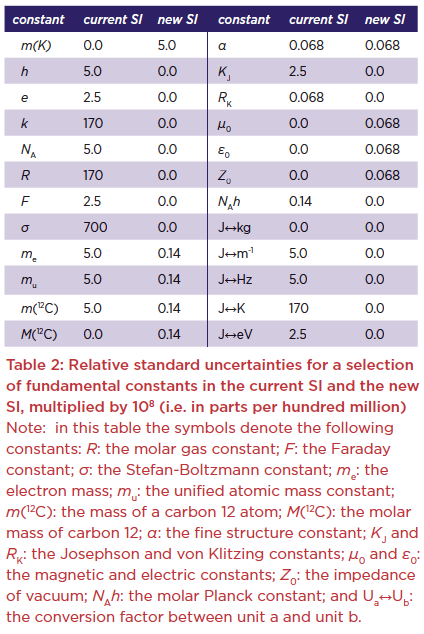
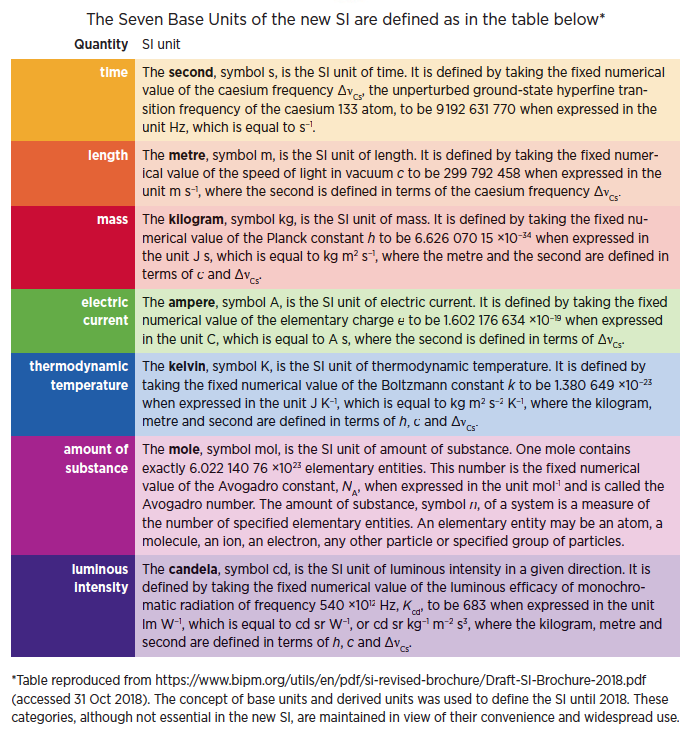
At a meeting of the General Conference on Weights and Measures (CGPM) held in Paris on 16 November 2018 a new and revised SI was approved, adapted for the 21st century.* These changes will be implemented on 20 May 2019, the next “World Metrology Day” and the day anniversary on which the Convention of the Metre was first signed in 1875. Of particular interest to chemists are the changes in the definition of the SI units kilogram and mole.
* See We Did It! Redefinition of SI adopted, video release by The BIPM, 20 Nov 2018


 In this context the well-known opening sentence of Jane Austen’s novel Pride and Prejudice may be adapted to read: “It is a truth universally acknowledged that a single man in possession of a good fortune, must be in want of a good set of units.” In the last four words of this sentence Jane Austen wrote “must be in want of a wife,” but we have substituted “in want of a good set of units.” Perhaps we need both!
In this context the well-known opening sentence of Jane Austen’s novel Pride and Prejudice may be adapted to read: “It is a truth universally acknowledged that a single man in possession of a good fortune, must be in want of a good set of units.” In the last four words of this sentence Jane Austen wrote “must be in want of a wife,” but we have substituted “in want of a good set of units.” Perhaps we need both!